Can standard Gibbs free energy be negative?
Índice
- Can standard Gibbs free energy be negative?
- What happens if Gibbs free energy is negative?
- Is Gibbs free energy always positive?
- Do Delta G become more negative?
- Is Delta S 0 at equilibrium?
- What happens when Delta G is negative?
- What exactly is Gibbs free energy?
- What if Delta S is negative?
- Why Gibbs free energy is called free energy?
- Does negative delta G mean spontaneous?
- What does Gibbs free energy tell us?
- Is Gibbs free?
- What is standard Gibbs free energy change?
- What is the equation for Gibbs free energy?
Can standard Gibbs free energy be negative?
Yes, the Gibbs free energy can be negative or positive or zero.
What happens if Gibbs free energy is negative?
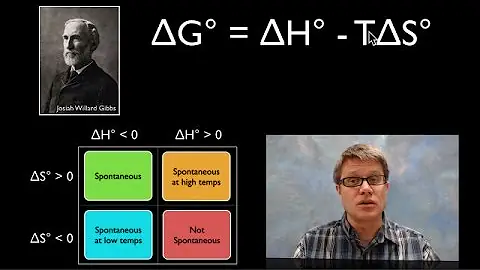
Gibbs free energy is a derived quantity that blends together the two great driving forces in chemical and physical processes, namely enthalpy change and entropy change. ... If the free energy is negative, we are looking at changes in enthalpy and entropy that favour the process and it occurs spontaneously.
Is Gibbs free energy always positive?
It is the entropy term that favors the reaction. Therefore, as the temperature increases, the TΔS term in the Gibbs free energy equation will begin to predominate and ΔG will become negative....Gibbs Free Energy.
| ΔH | ΔS | ΔG |
|---|---|---|
| positive | negative | always positive |
Do Delta G become more negative?
G=-rTlnKeq - so as the temperature increases, the delta G usually gets more (-), or spontaneous. Changes in temperature can make G more negative and the reaction more spontantous. If in the above equaiton S is (-), then increasing temperature won't make it more spontaneous (because -TS) term becomes more positive.
Is Delta S 0 at equilibrium?
If ΔG < 0, then K > Q, and the reaction must proceed to the right to reach equilibrium. If ΔG > 0, then K < Q, and the reaction must proceed to the left to reach equilibrium. If ΔG = 0, then K = Q, and the reaction is at equilibrium.
What happens when Delta G is negative?
Therefore, if ∆G is a negative number, the reaction favors products.
What exactly is Gibbs free energy?
The Gibbs free energy ( , measured in joules in SI) is the maximum amount of non-expansion work that can be extracted from a thermodynamically closed system (one that can exchange heat and work with its surroundings, but not matter).
What if Delta S is negative?
A negative delta S corresponds to a spontaneous process when the magnitude of T * delta S is less than delta H (which must be negative). delta G = delta H - (T * delta S). A negative delta S would mean that the products have a lower entropy than the reactants, which is not spontaneous by itself.
Why Gibbs free energy is called free energy?
Why is energy 'free'? ... This happens because the reaction gives out heat energy to the surroundings which increases the entropy of the surroundings to outweigh the entropy decrease of the system.
Does negative delta G mean spontaneous?
Reactions with a negative ∆G release energy, which means that they can proceed without an energy input (are spontaneous). In contrast, reactions with a positive ∆G need an input of energy in order to take place (are non-spontaneous).
What does Gibbs free energy tell us?
Gibbs free energy is a measure of the potential for reversible or maximum work that may be done by a system at constant temperature and pressure. It is a thermodynamic property that was defined in 1876 by Josiah Willard Gibbs to predict whether a process will occur spontaneously at constant temperature and pressure.
Is Gibbs free?
In thermodynamics, the Gibbs free energy (IUPAC recommended name: Gibbs energy or Gibbs function; also known as free enthalpy to distinguish it from Helmholtz free energy ) is a thermodynamic potential that can be used to calculate the maximum of reversible work that may be performed by a thermodynamic system at a constant temperature and pressure ( ...
What is standard Gibbs free energy change?
The standard Gibbs free energy of formation of a compound is the change of Gibbs free energy that accompanies the formation of 1 mole of that substance from its component elements, at their standard states (the most stable form of the element at 25 °C and 100 kPa).
What is the equation for Gibbs free energy?
The equation for Gibbs free energy is: G = H – TS. where G is Gibbs free energy, H is enthalpy, T is temperature, and S is entropy. Helmholtz free energy is energy that may be converted into work at constant temperature and volume.